

The formula for hydrosulfuric acid, also known as hydrogen sulfide, is H2S. This molecule has two lone pairs and three bound pairs, according to the ClF3 Lewis structure. What is CLF3 molecular geometry?ĬlF3 has a T-shaped molecular geometry and a trigonal bipyramidal electron geometry. it is neutral, but when it is made into an aqueous solution, it releases the hydronium ions H+ and S2- ions. In its gaseous form, H2S is neither acidic nor basic, i.e. The structure is a bent structure with bond angles of 92.1 degrees. Hydrogen sulfide is a polar molecule with a single bond between two hydrogen and sulfur atoms. Explain Hydrogen sulfide Lewis Structure in simple words Some examples of polar molecules are water (H 2O), Ethanol, Ammonia, and SO 2 (Sulfur Dioxide). The slight electrical charges on dissimilar atoms are called partial charges, and the presence of partial charges signifies the occurrence of a polar bond. Specifically, it is found that bonds between atoms of different elements are electrically inequivalent.įor instance, in hydrogen chloride, the H atom is slightly positively charged whereas the Cl atom is slightly negatively charged.

The distribution of electrical charge over the atoms joined by the bond causes polarity. Some of the frequently asked questions are given below 1. More Interesting Topics Molar Mass of Acetic Acid| Easy-Explanation Concentration Gradient Definition Sodium Phosphate – Formula, Structure, Types, and Uses Sulfurous Acid| Formula & Lewis Structure Valence Electrons in Nitrogen Is Nh3 Polar? Frequently Asked Questions (FAQs)
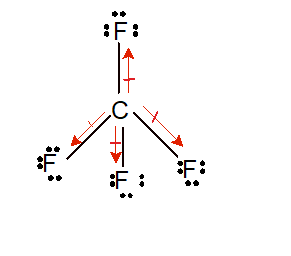
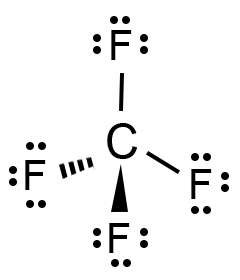
In the case of a symmetric structure, the dipole vectors on each molecule cancel each other, resulting in the nonpolar nature of the molecule. A molecule is polar if the structure of that molecule is not symmetrical. Polarity is determined by electronegativity. The molecular geometry of hydrogen sulfide is polar but the bonds are not polar. H2S is a slightly polar molecule because of the small difference in electronegativity values of hydrogen (2.2) atoms and sulfur (2.58) atoms. Farmers use hydrogen sulfide as an agricultural disinfectant and it is found in some cutting oils, which are coolants and lubricants designed specifically for metalworking and machining processes.H 2S is used primarily to produce sulfuric acid and sulfur.It plays a vital role in signaling pathways in the human body.It is the main precursor of elemental sulfur.B., Thermodynamic Properties of Individual Substances, Fouth Edition, Hemisphere Pub.
#CF4 MOLECULAR GEOMETRY FREE#
K Kuchitsu(ed) "Structure of Free Polyatomic Molecules - Basic Data" Springer, Berlin, 1998 TN Olney, NM Cann, G Cooper, CE Brion, Absolute scale determination for photoabsorption spectra and the calculation of molecular properties using dipole sum-rules, Chem. Please address comments about this page to Jones, C Kennedy, S Ekberg "Potential Constants of CF4" J. NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites.įurther, NIST does not endorse any commercial products that may be mentioned on these sites. There may be other web sites that are more appropriate for your purpose. No inferences should be drawn on account of other sites being referenced, or not, from this page. We have provided these links to other web sites because they may have information that would be of interest to you. You are here: Experimental > One molecule all propertiesĮxperimental data for CF 4 (Carbon tetrafluoride)Īrcton 0 Carbon fluoride Carbon fluoride (CF4) Carbon tetrafluoride F 14 FC 14 Freon 14 Halocarbon 14 Halon 14 Methane, tetrafluoro- Perfluoromethane R 14 Refrigerant 14 Tetrafluoromethane ĭistances (r) in Å, angles (a) in degrees, dihedrals (d) in degreesĮxperimental Bond Angles (degrees) from cartesians atom1Įxamples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bondīy selecting the following links, you may be leaving NIST webspace.


 0 kommentar(er)
0 kommentar(er)
